Abstract
The management of refractory myeloma patients (pts) to anti-CD38-based therapies is challenging despite the increasing number of treatment options. In addition, the use of anti-CD38 monoclonal antibodies (MoAbs) in earlier lines of treatment increases the number of refractory pts to these drugs. Management of such pts is based either in the use of drugs with new mechanisms of action (if available) or in the retreatment with same agents or drug classes used in prior lines. Herein, we describe the outcomes of pts who failed on anti-CD38-containing therapy.
The analysis included 183 pts with RRMM who progressed during therapy with a daratumumab- or isatuximab-based regimens (index therapy) and who received further therapy. Pts were treated in a single center (Department of Clinical Therapeutics, University of Athens, Greece) and started anti-CD38 therapy from 1/1/2015 to 31/12/2021.
The pts received index anti-CD38 therapy after a median of 2 prior lines of therapy (range 1-10). Their median age was 68 years (range 35-89); 38% had prior ASCT, 61% were PI-, 71% lenalidomide- and 29% pomalidomide-refractory. Anti-CD38 was given as monotherapy (+/- dexa) in 50% of pts, while its combination with IMiD was given in 25% of pts and with PI in 25% of them. On ITT, ORR was 55% (CR: 5%, VGPR: 25%, PR:25%). Median PFS on anti-CD38 therapy for the whole cohort was 6.4 months; it was 5.5 months for anti-CD38 +/- dexa vs 7.4 months for anti-CD38 triplets (p=0.055).
Post anti-CD38 progression (median 3 prior lines) all pts were PI and lenalidomide exposed, 82% were PI refractory, 75% lenalidomide refractory and 46% pomalidomide refractory. Treatment post anti-CD38 failure included PI-based regimens in 40% of pts (23% carfilzomib-based), 30% pomalidomide containing, 23% anti-CD38 triplet combinations, 15% belantamab mafodotin, 5% selinexor (+/-PI); 50% received a triplet and 50% a doublet. On ITT, ORR in the post anti-CD38 line was 43% and the median PFS was 6.4 months; PFS was 5.5 months for pts that had progressed on anti-CD38+/-dexa, post-anti-CD38 and 7.4 months for those progressing on anti-CD38-combinations (p=0.195). Given the high rates of PI and lenalidomide resistance, only pomalidomide sensitivity was associated with better PFS post anti-CD38, which remained poor (median 7.5 vs 5.2 months, p=0.007). Median PFS in the post anti-CD38 line was 4 months for those who received anti-CD38 containing regimens, 6.4 months for PI-based regimens (for carfilzomib-containing 6.7 months), 4.5 months for pomalidomide-based, 6 months for triplets (vs 6.8 for doublets) while it was 9.1 months (95% CI 4.5-13.9) for belantamab mafodotin and 3.7 months in the small group of selinexor treated pts.
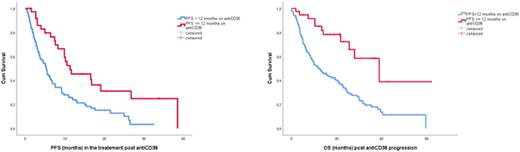
A PFS ≥12 months during index anti-CD38 therapy was the most important prognostic factor for PFS post anti-CD38 failure (median PFS post anti-CD38 of 11.3 months vs 5.2 months, p=0.001). This prognostic effect was independent of the number of prior lines, type of treatment or pomalidomide resistance. Notably, the use of anti-CD38 combinations was associated with a PFS of 11.6 months in pts with a PFS ≥12 months during initial anti-CD38 therapy. The median OS post anti-CD38 progression was 17.6 months; 12.8 vs 22.5 months for pts who had progressed on antiCD38+/-dexa vs antiCD38 combinations respectively (p=0.327), The median OS post index therapy was 16.6 months for those who received anti-CD38 containing regimens, 22.9 months for PI-based regimens (for carfilzomib 22.7 months), 20.9 months for pomalidomide-based therapies, 21 months for triplets (vs 19.3 for doublets), 24.5 months for belantamab and 30 months for the small group of selinexor-treated pts. OS post anti-CD38 was 39 months for those with PFS ≥12 months on index antiCD38 therapy (vs 12 months, p<0.001) and this was the only important prognostic factor.
In conclusion, outcomes after progression to anti-CD38 therapy are poor, despite recent therapeutic advances. Drugs with new mechanisms of action may improve PFS, but their use needs to be optimized, probably in the context of triplet combinations. Recycling prior therapies, even anti-CD38 drugs, may be beneficial especially for pts who had prolonged remission during the index anti-CD38 treatment and could be useful as a bridge to other options. In this subgroup of pts, maintaining an antiCD38 backbone could be a valid option but participation in clinical trials should be highly encouraged.
Disclosures
Kastritis:Janssen: Honoraria, Research Funding; Genesis Pharma: Honoraria; GSK: Honoraria; Pfizer: Honoraria, Research Funding; Takeda: Honoraria; Amgen: Honoraria, Research Funding. Gavriatopoulou:Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Janssen Cilag: Honoraria; Sanofi: Honoraria; Genesis Pharma: Honoraria. Terpos:BMS: Honoraria; EUSA Pharma: Honoraria, Other: Travel expenses; Genesis: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Novartis: Honoraria; Takeda: Honoraria, Other: Travel expenses, Research Funding; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Amgen: Honoraria, Other: Travel expenses, Research Funding. Dimopoulos:Takeda: Honoraria; BMS: Honoraria; Beigene: Honoraria; Amgen: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal